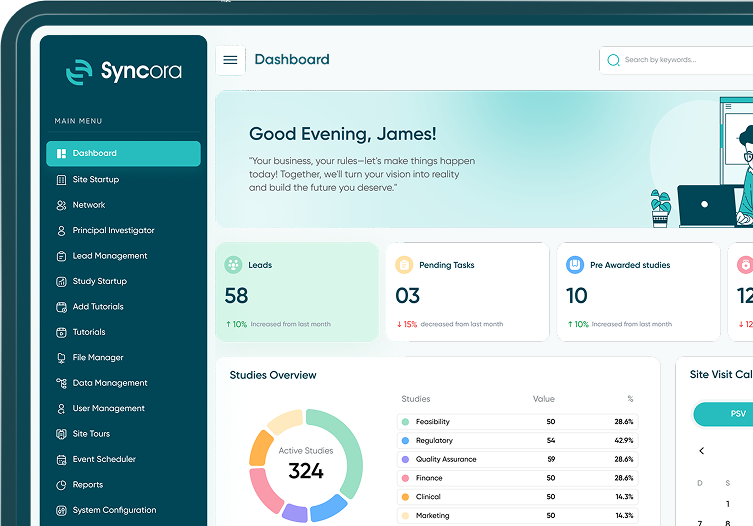
Syncora simplifies every step of the study startup process, empowering teams to manage site startup, tasks, and compliance seamlessly—all within one powerful platform.

Trusted by leading businesses around the world




Streamlined Study Startup Workflow
Take the complexity out of study startups with Syncora’s intuitive workflow management. Our platform simplifies tasks, tracks progress and ensures nothing falls through the cracks.
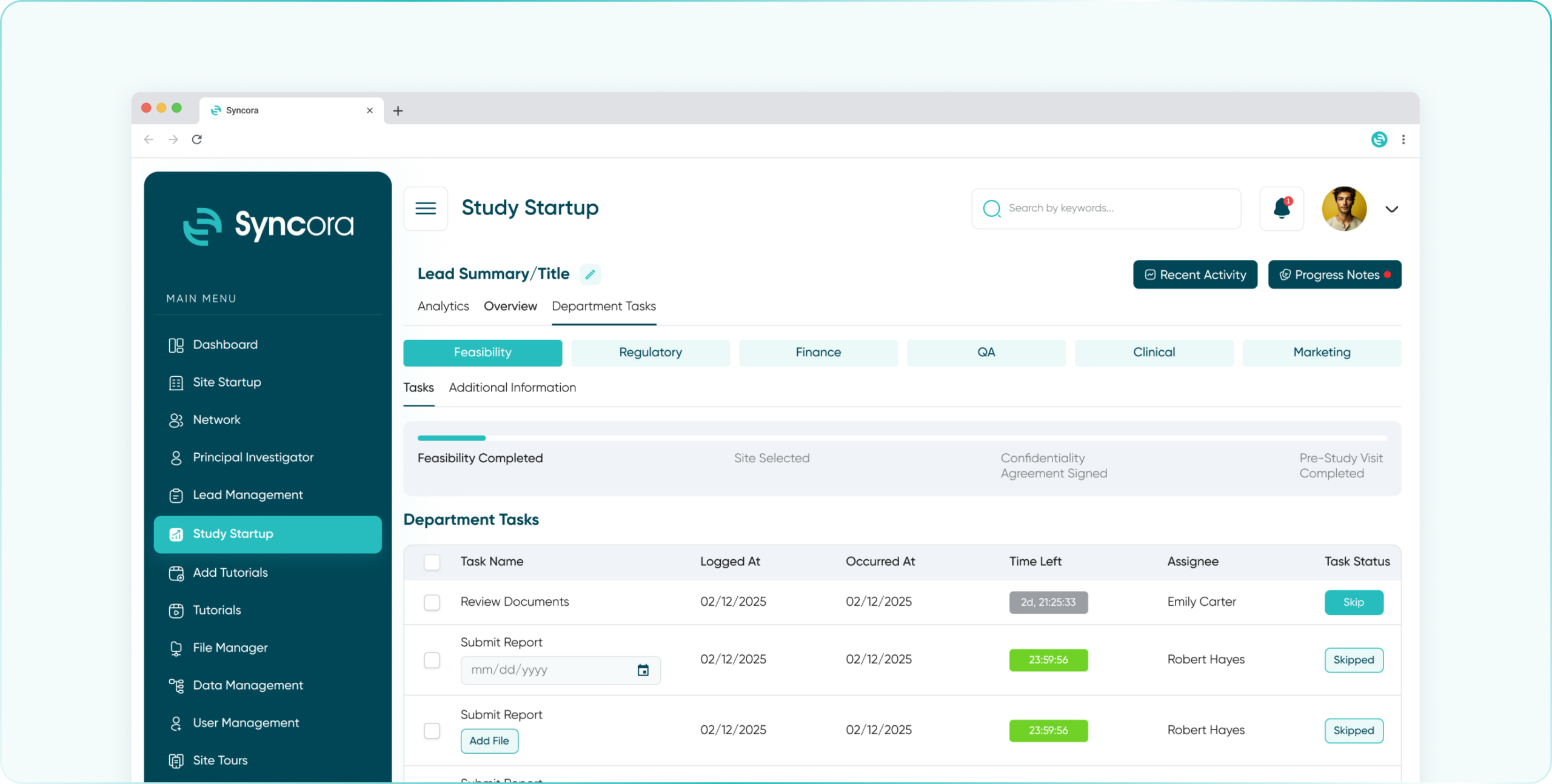
Efficient Site Startup Management
Empower your sites to hit the ground running with Syncora. From documentation to site readiness, our platform offers everything you need to accelerate site activation and ensure compliance at every step.
Empowering Principal Investigators (PIs)
Support your Principal Investigators with the tools they need to succeed. Syncora keeps PIs informed, organized, and engaged throughout the study lifecycle, ensuring seamless collaboration and top-notch performance.
Empower everyone, on every team
- Feasibility Department
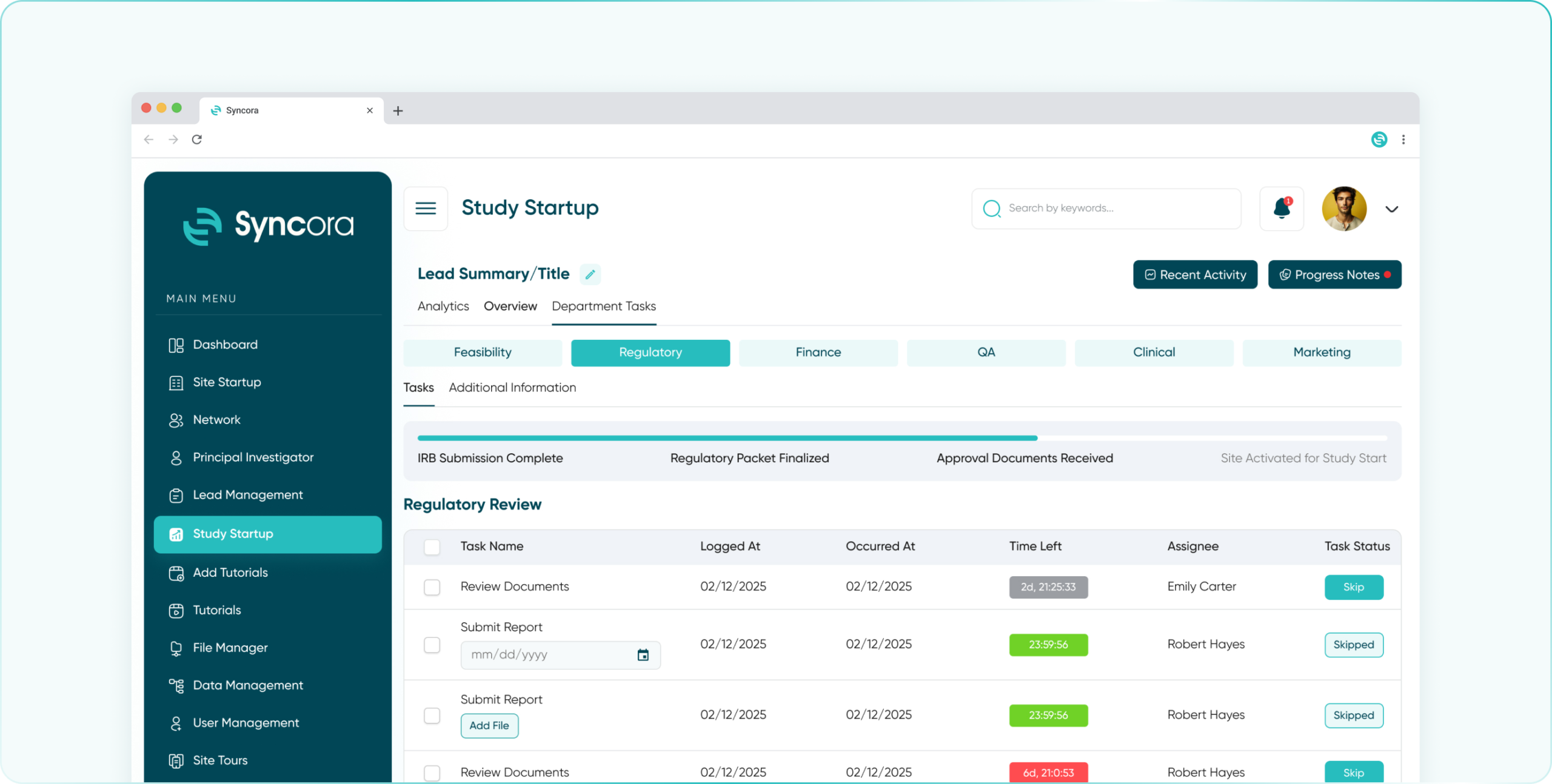
- Regulatory Department
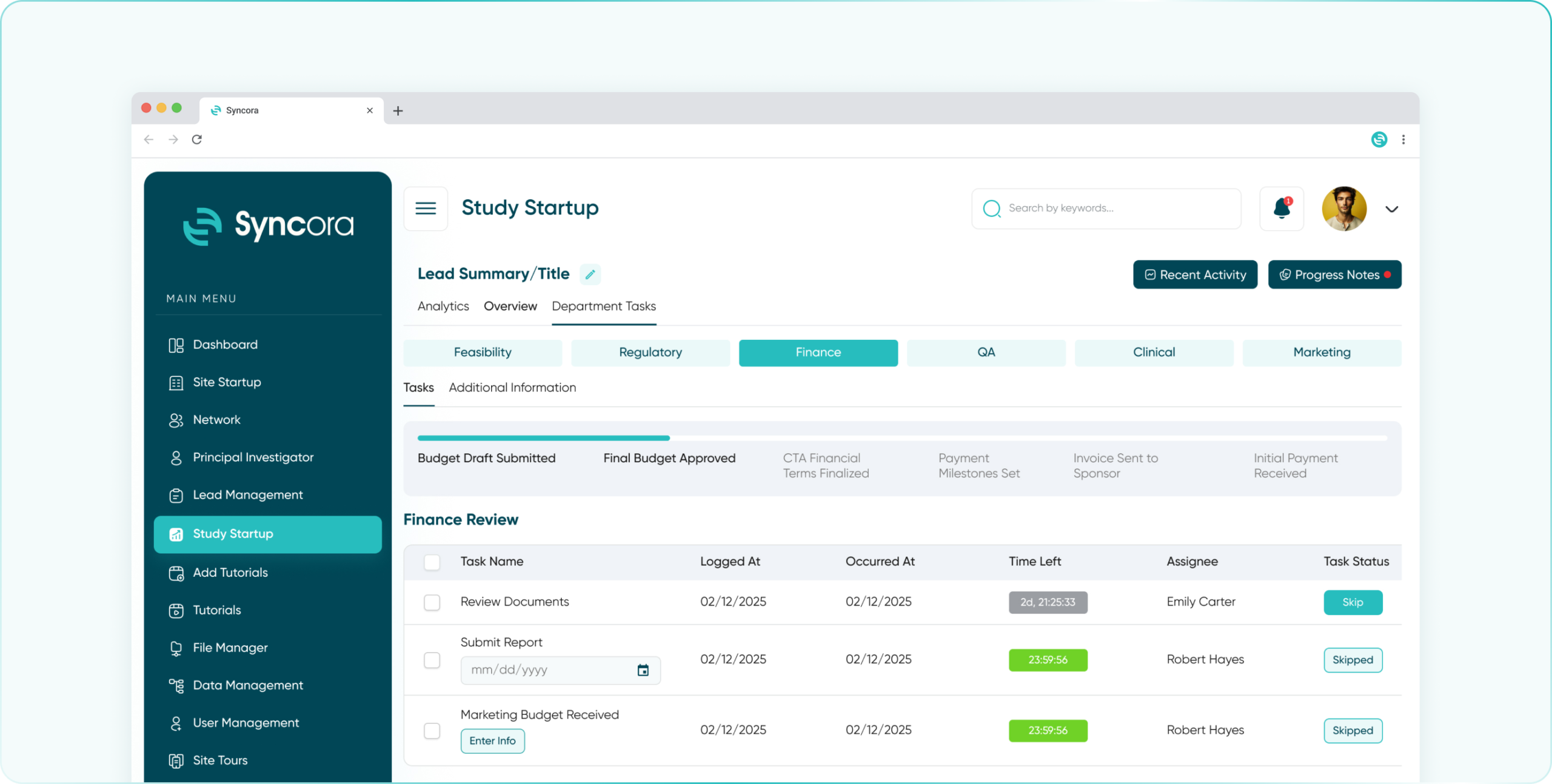
- Finance Department
- QA Department
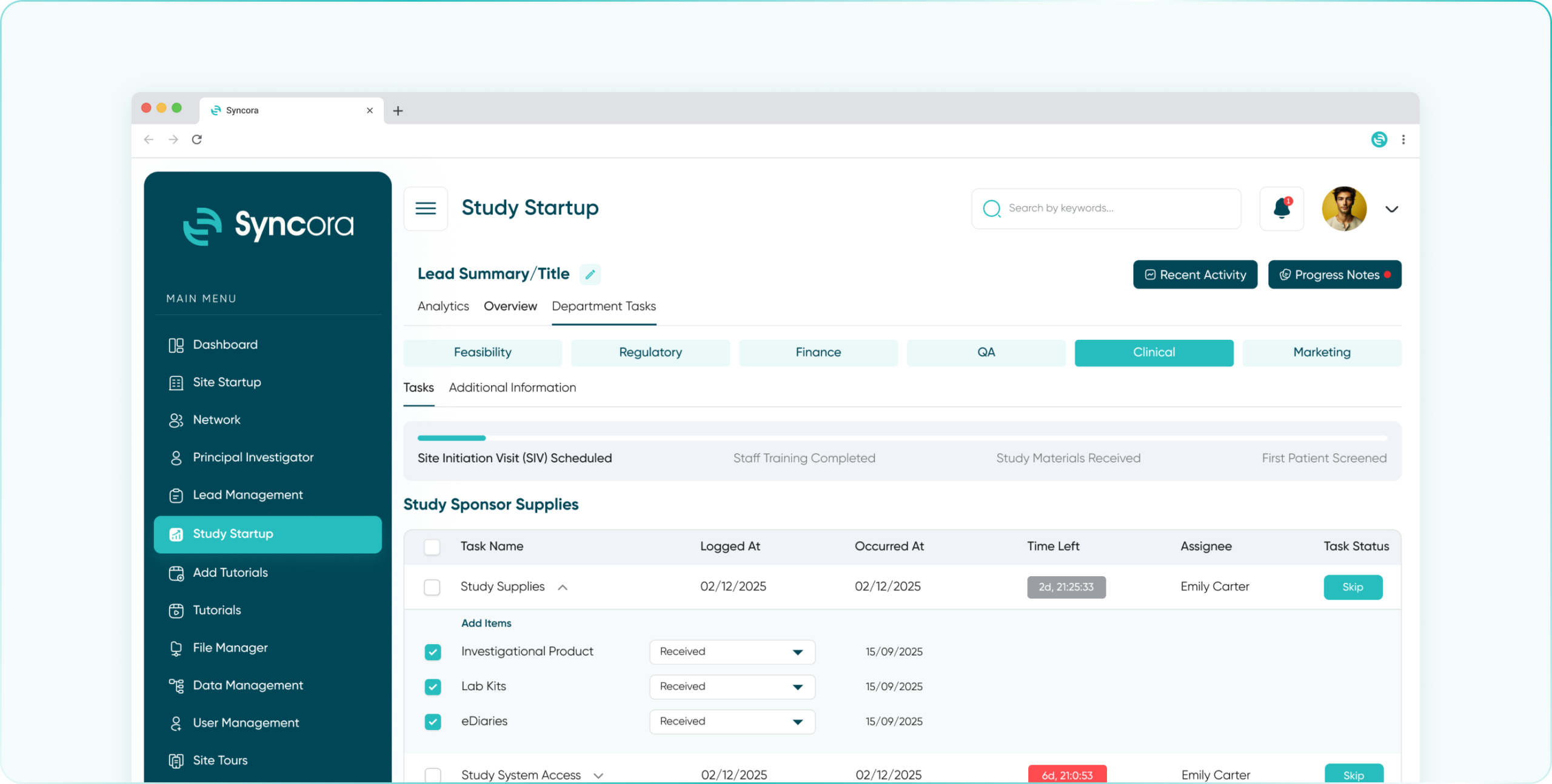
- Clinical Department
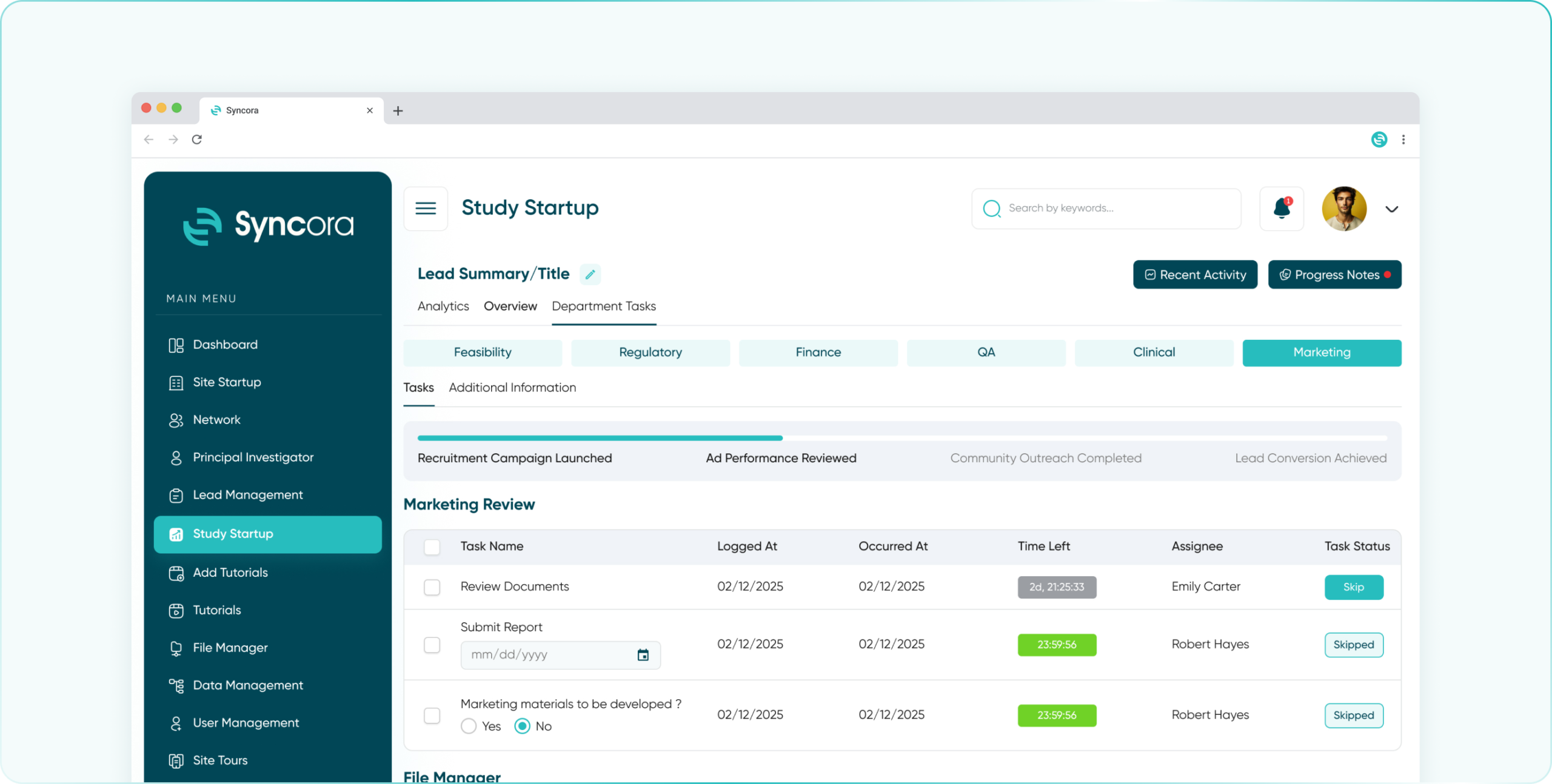
- Marketing Department
Empower your team with tools that streamline site selection and generate data-driven insights, ensuring your trials start on the right foot.
Simplify compliance with Syncora’s centralized platform, enabling seamless regulatory document management, approval tracking, and adherence to SOPs.
Optimize budgeting and payments with real-time financial tracking, automated invoicing, and transparency for every dollar spent on clinical trials.
Ensure quality at every step with Syncora’s robust tools for monitoring, audits, and compliance tracking to uphold your trial’s integrity.
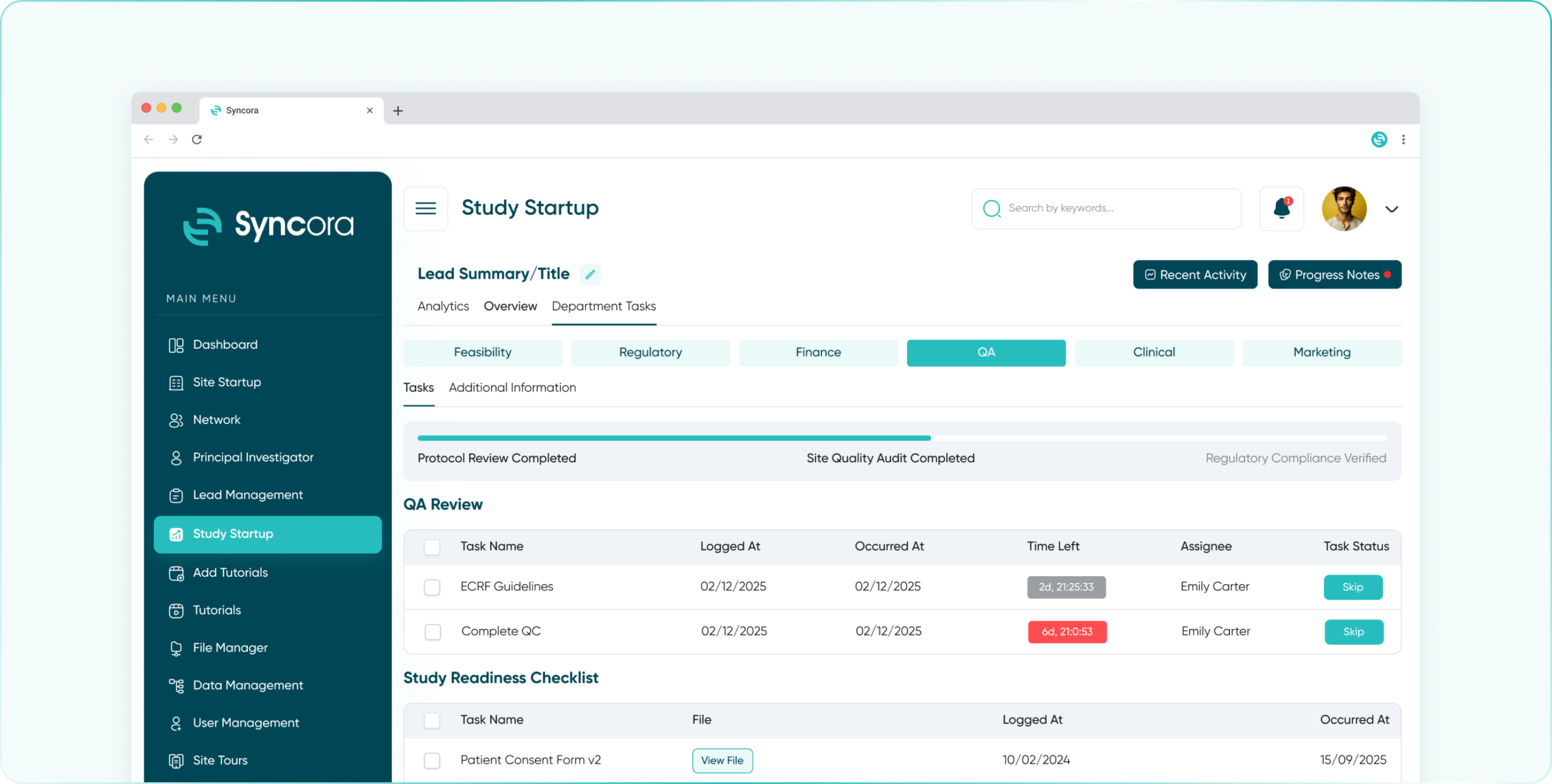
Streamline study oversight with a comprehensive dashboard, task automation, and real-time access to site performance and patient data.
Boost participant recruitment and engagement with marketing tools designed to create, track, and optimize outreach campaigns.
Numbers That Drive
Clinical Excellence
See What Our Customers

Are Saying About Syncora
Explore How Syncora is
Shaping the Future of
Clinical Research
Join us on a journey through our groundbreaking work in clinical trials, innovative solutions, and the people driving progress in healthcare.
Stay Informed with the
Latest from Syncora
Cost Savings
User Experience
24/7 Support
Safe & Secure
why book a demo with syncora?
- Stay ahead with the latest in study startup innovations.
- Hear directly from Syncora’s trusted research partners.
- Get smarter, faster, and simpler trial management strategies.