Syncora simplifies study startup management with innovative, value-driven features designed to optimize processes, improve collaboration, and drive successful outcomes.
Smart Tools, Smarter Clinical Trial Startup
Syncora simplifies study startup management by offering a secure, intelligent, and user-friendly solution that optimizes site operations and accelerates study startup.
Networks
Syncora’s Networks feature centralizes communication and resource-sharing, making it easy to manage single sites or large vendor networks while ensuring consistent compliance and smooth coordination.

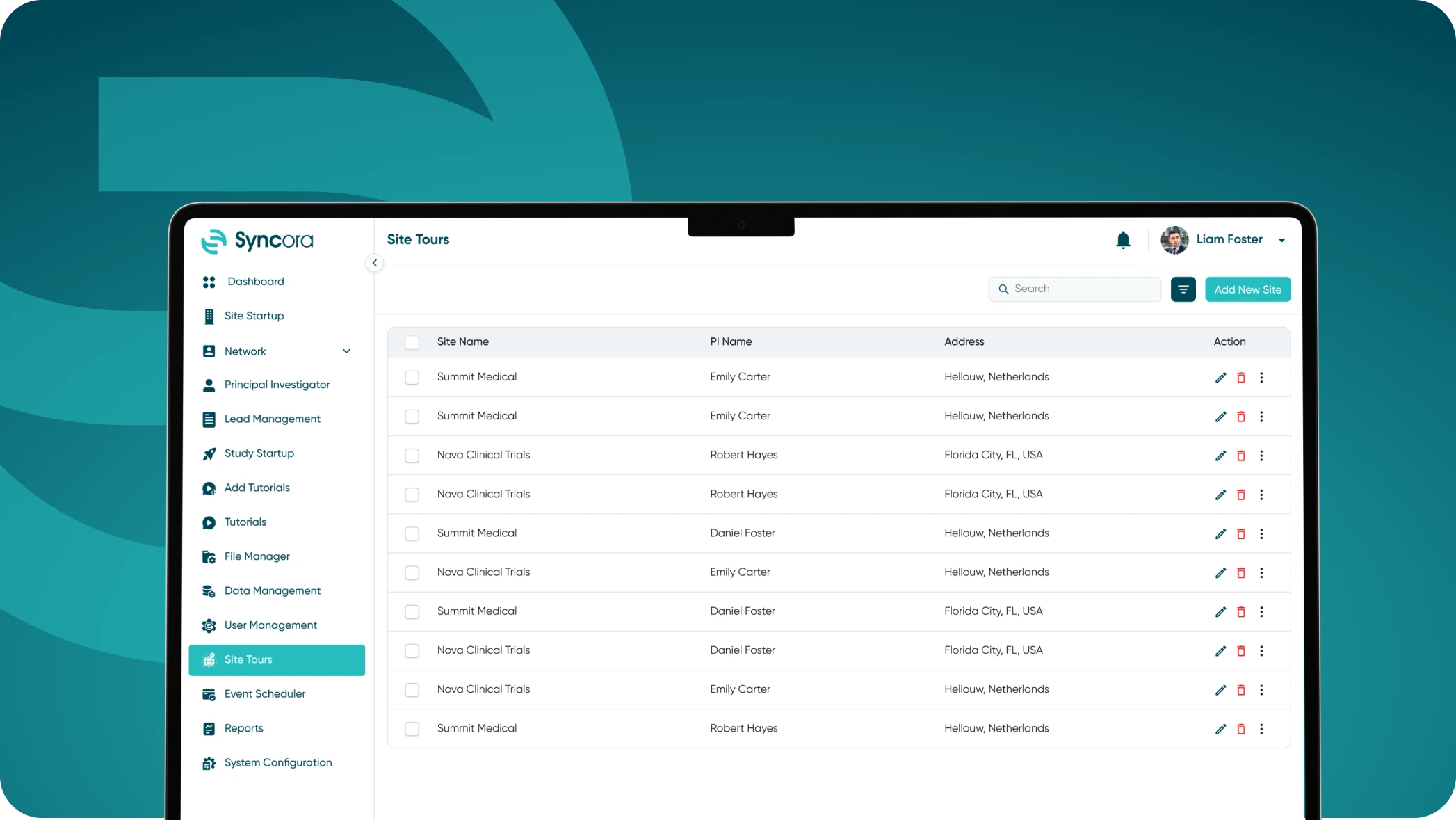
Virtual Tour
Bring trial planning to life with Syncora’s Virtual Tour feature. Visualize site readiness, optimize space allocation, and address potential gaps before trial initiation. This feature ensures all teams are aligned, and sites are prepared to deliver successful studies without unexpected delays.
21 CFR Part 11
Stay compliant with FDA regulations effortlessly. Syncora’s 21 CFR Part 11 feature ensures electronic records and signatures meet industry standards, providing an audit-ready environment that maintains data integrity and regulatory adherence.

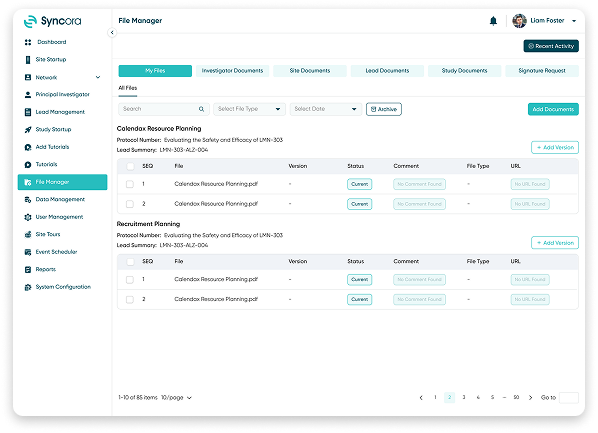
File Manager
Organize, share, and secure trial documents with Syncora’s File Manager. With built-in version control, real-time updates, and role-based access, managing documents has never been simpler. Eliminate duplication and boost efficiency by keeping your team in sync.
Launch Sites Faster with Smarter Tools
Syncora’s Site Startup tools simplify and accelerate the study startup process. From site selection to activation, Syncora ensures every step is seamless and efficient.
Syncora: Built for Flexibility
Syncora’s Site Startup streamlines site selection and activation with customizable workflows, real-time updates, and automated document management—ensuring timely study launches.


Automation at Every Step
Syncora eliminates redundancies by automating essential tasks like document submission, compliance tracking, and site activation workflows. Focus on strategic decisions while Syncora handles the operational complexities for you.

Seamlessly Integrated for Efficiency
Syncora seamlessly integrates with your existing systems, allowing effortless data transfer and real-time collaboration. Unify site selection, onboarding, and activation or deactivation processes with a single, powerful solution.
Empower Every Team, Every Step of the Way
Optimize operations with centralized documents, real-time tracking, and automated workflows. Syncora streamlines collaboration and keeps research sites audit-ready.

Strengthen coordination with tools that standardize processes, enhance transparency, and ensure quality across all sites.
Streamline trials with easy data access, automated scheduling, and real-time insights. Syncora helps PIs stay compliant and improve patient outcomes.

Elevate trial quality with advanced monitoring, streamlined reporting, and early risk detection—ensuring compliance and continuous improvement.




PSV-SIV Scheduler - Plan Smarter, Activate Faster
Schedule pre-site visits (PSV) and site initiation visits (SIV) effortlessly with Syncora’s PSV-SIV Scheduler. Ensure every milestone is planned and executed seamlessly.

Turn Data into Actionable Insights
Transform data into insights with Syncora’s advanced reporting tools. Make informed decisions with real-time reports that provide clarity and transparency.

Automated Reporting for Study Startup Excellence
Syncora’s Reporting feature automates data collection and analysis, delivering customized reports on key performance indicators. Quickly identify bottlenecks, improve resource allocation, and ensure your trials run efficiently from start to finish.
Study Startup Tracking
Syncora’s tracking tools offer real-time updates on timelines, tasks, and resources—keeping your study startup on schedule, transparent, and efficient.
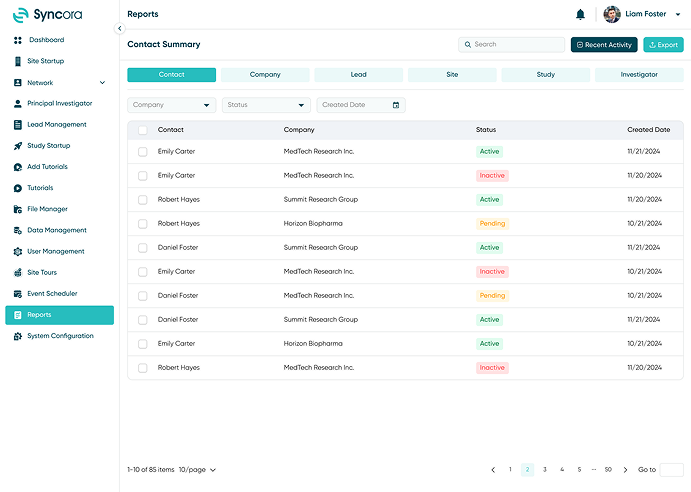
Reports
Syncora’s reporting tools turn data into actionable insights. With customizable dashboards and detailed metrics, you can focus on the key performance indicators that matter most. Ensure accountability and make informed decisions with clear, comprehensive reports tailored to your study needs.
Comprehensive Management, Simplified
Manage every aspect of your trial efficiently with Syncora’s tools for lead, PI, and site management.

Lead Management:
Syncora’s Lead Management centralizes study lead tracking, enabling faster conversions through tailored communication and real-time follow-ups.

PI Management:
Syncora simplifies Principal Investigator management with tools for credential tracking and performance monitoring, ensuring they’re fully supported to excel in your studies.

Site Management:
Syncora’s Site Management tools help you track activity, ensure compliance, and streamline communication for efficient, aligned trial operations.













